IKS03
IKS03 is currently in Phase 1 clinical trials for B-cell lymphomas.
IKS03 has been designed to deliver high anti-cancer activity with good patient tolerability. The ADC benefits from tumor-selective release and activation of ultra-potent PBD payloads for an enhanced TI.
Preclinical studies have demonstrated class leading efficacy across a wide range of B-cell cancer models and improved safety over in-clinic comparators. Phase 1 recruitment is ongoing at sites in Australia, the United States and Canada
IKS03 development status
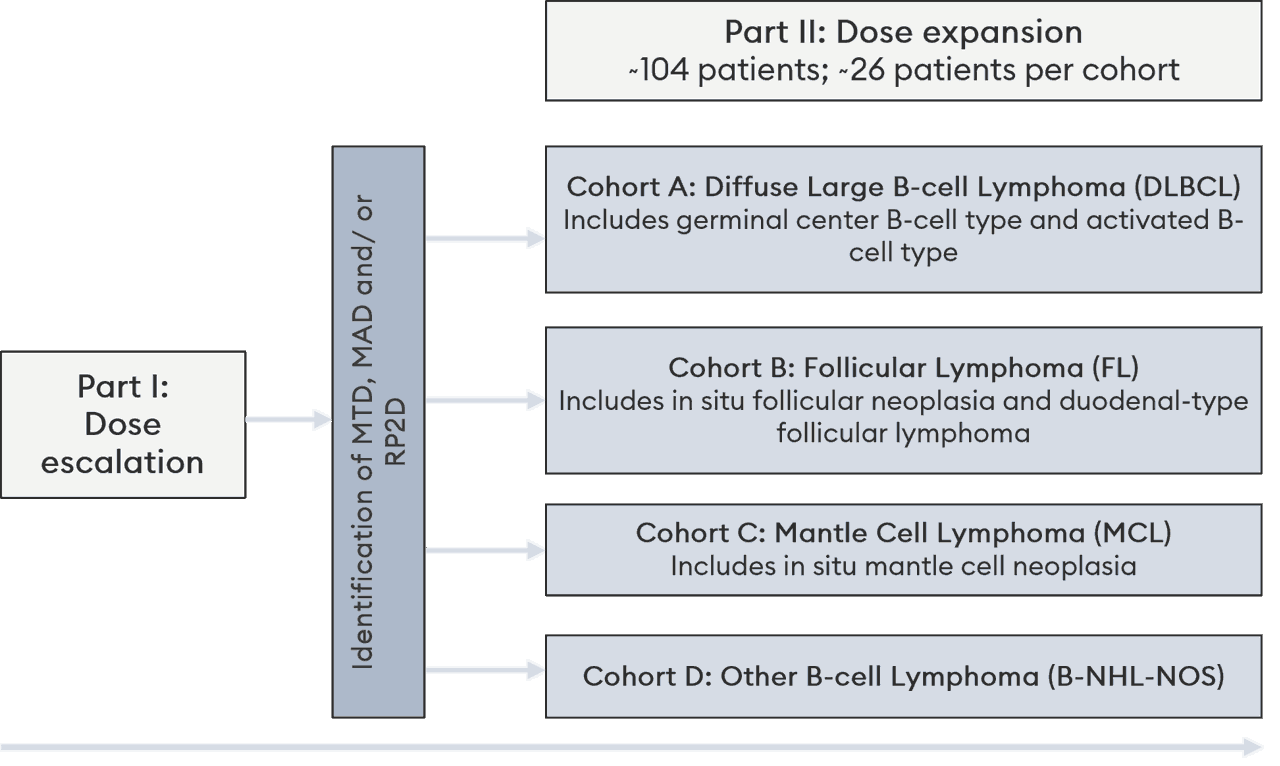
Clinical trial overview
Part I is a dose escalation 3+3 design study, with a primary objective of safety (MTD).
Part II is a dose expansion study in specified indications, with a primary objective of Objective Response Rate (ORR)
The study is being conducted in centers in North America and Australia with a projected trial size of 140 patients
Site locations
North America
University of Maryland: Baltimore, Maryland, United States 21201
McGill University/ Jewish General Hospital: Montreal, Quebec, Canada H3T 1E2

Australia
Sir Charles Gairdner Hospital/Linear Clinical Research,
Hospital Ave, Nedlands,
Perth, WA 6009, Australia
Royal Hobart Hospital, 48 Liverpool St,
Hobart TAS 7000, Australia

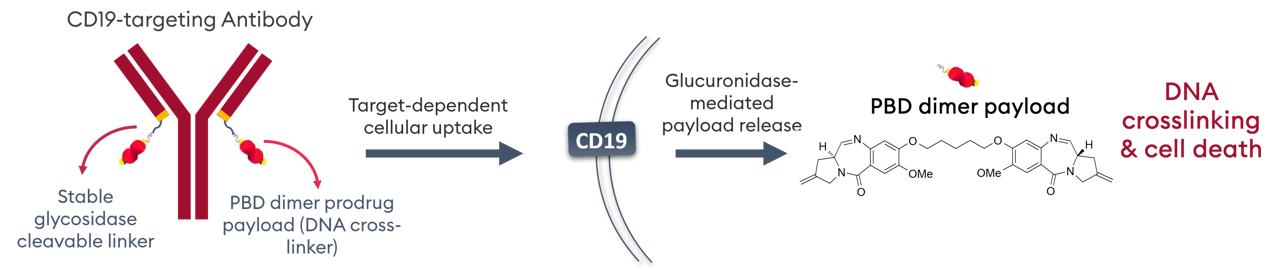
Differentiation by design
A beta-glucuronide linker is used for tumor-selective payload release, whilst a second beta-glucuronide moiety improves hydrophilicity and plasma stability of the PBD prodrug.
Payload release and ADC activation requires processing by beta-glucuronidase, an enzyme overexpressed in cancer cell lysosomes, active only at acidic pH and with expression in normal tissues.
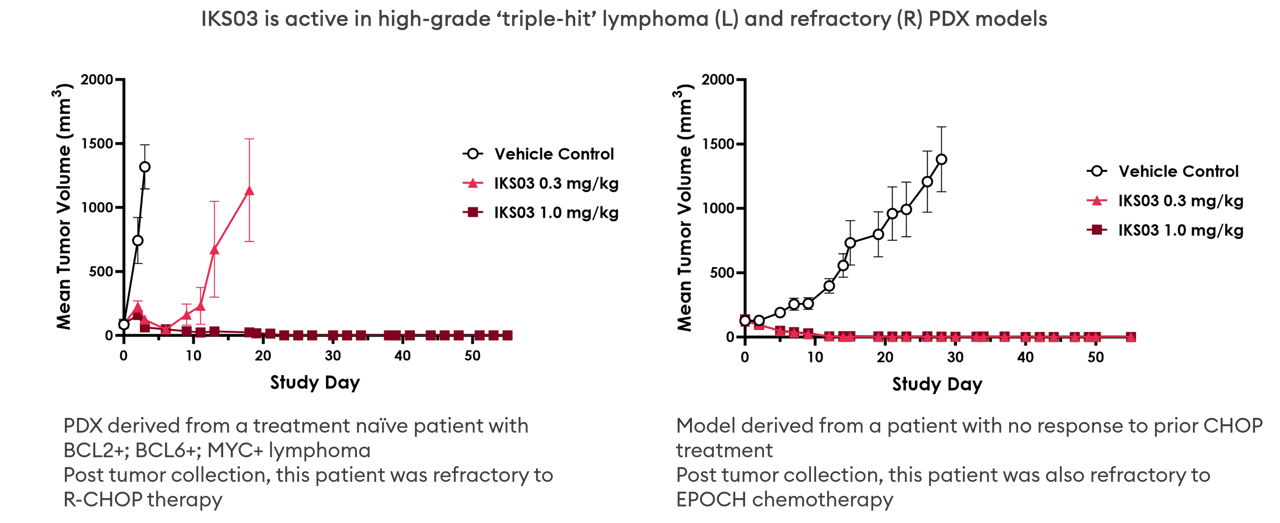
Class leading profile
Compared with the in-clinic comparator Zynlonta loncastuximab tesirine), IKS03 was significantly more active at lower doses after single-dose administration, with an HNSTD (in NHPs) that was approximately twofold higher.
IKS03’s advanced ADC design improves upon the high efficacy of DNA crosslinkers whilst not being associated with delayed toxicity, thus significantly increasing therapeutic index.
In addition, IKS03 is efficacious in high-grade 'triple hit', refractory and MYC-amplified lymphoma PDX models in vivo.
IKS03 has Best in class TI for CD19-targeting therapies
| Drug | Payload |
MED (mouse CR) |
Preclinical Therapeutic Index* |
| Zynlonta (loncastuximab tesirine) | PBD | 0.6-1.0 mg/kg | <1 |
| IKS03 | proPBD | ≤0.3 mg/kg | >5 |
| Polivy | MMAE | 2.5 mg/kg | <1.2 |
*TI = monkey HNSTD/mouse CR: IKS03 is well tolerated at 1.5mg/kg in monkeys
Pipeline prioritization strategy
Iksuda only progresses ADC programs which meet our design criteria to clinical assessment. For programs directed to known ADC targets, with in-clinic and/ or on-market ADCs available, raising TI to a clinically meaningful degree is prerequisite in our decision-making.

Downloads
Keep informed
Sign up for our latest programme news and insights