IKS04
IKS04 is the first 'ultra-low DAR' ADC in development for solid tumors
IKS04 is currently in late preclinical development with IND anticipated by Q4 2024/ Q1 2025. It represents a new approach for the treatment of GI cancers and is the only CA242-directed ADC in development.
Development status
Differentiation by design
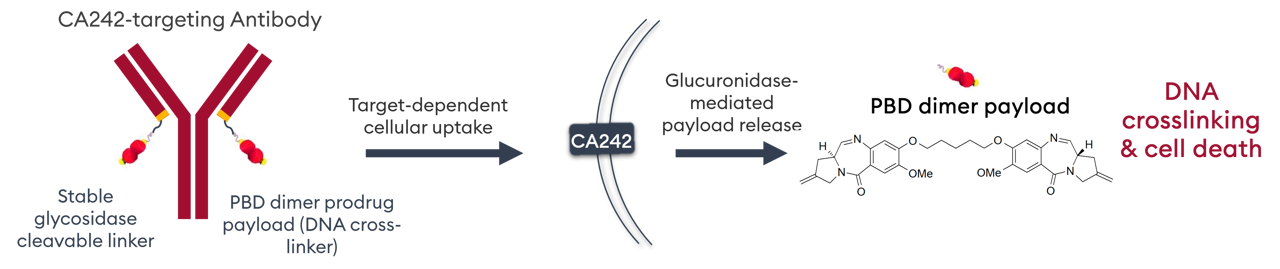
A beta-glucuronide linker is used for tumor-selective payload release, whilst a second beta-glucuronide moiety improves hydrophilicity and plasma stability of the PBD prodrug.
Payload release and ADC activation requires processing by beta-glucuronidase, an enzyme overexpressed in cancer cell lysosomes, active only at acidic pH and with low expression in normal tissues.
Creating a perfect storm of positivity for cancer patients
However, such payloads need to be delivered precisely and be associated with a suitable safety & efficacy profile. Iksuda’s ADCs utilize tumor-selective activation and release of payloads, currently through the incorporation of beta-glucuronide (BG) linkers and triggers.
Our IKS03 program demonstrates the potential of the PBD prodrug approach as a suitable option for hard-to-treat solid tumors. This ADC contains a highly potent, talirine-like PBD, safely delivered via the BG-driven mechanism. In preclinical studies, tumor-selective delivery drives class leading efficacy and safety, resulting in significantly enhanced TI. ADC design is also associated with bystander effect, suggesting that solid tumors with heterogeneous/ low expression may also benefit.
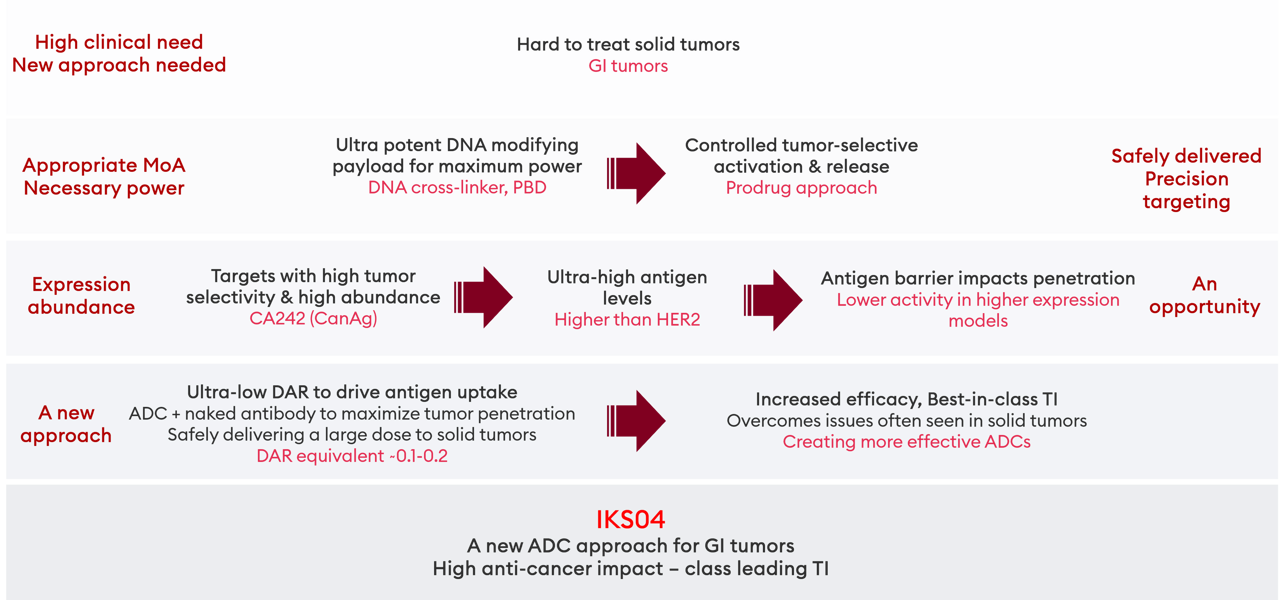
For our IKS04 program, Iksuda considered antigen targets for GI tumors which are associated with high expression abundance and high tumor selectivity: CA242 (CanAg) represents the most appropriate choice.
IKS04 is therefore comprised of an anti-CanAg antibody engineered for site-specific conjugation of a PBD prodrug, where a BG linker is used for tumor-selective payload release, and a second BG moiety improves hydrophilicity and provides added reassurance against unwanted binding to proteins in plasma. β-glucoronide is overexpressed in cancer cell lysosomes, active only at acidic pH and with low expression in normal tissues.
Ultra-low DAR approach
Due to the high abundance in CanAg-high expressing models – higher than that seen with HER2 – an antigen barrier lowers tumor penetration and ADC efficacy compared with CanAg-low models. CanAg represents a perfect target for an ultra-low DAR approach.
In preclinical studies, IKS04, administered as a single dose, induces impressive efficacy across a range of CanAg-expressing models and GI tumors – significantly higher than the clinical benchmark ADC cantuzumab ravtansine, (a CanAg-directed ADC which was suspended during clinical trials by Immunogen due to a sub-optimal profile) in all xenografts tested.
However, activity is highest in low-expressing models and lowest in high-expressing models suggesting that high expression abundance impacts tumor penetration.
For this antigen, the high CanAg-expressing Colo205 (colorectal adenocarcinoma) and SNU16 (gastric adenocarcinoma) models, the copies/ cell are ultra-high compared with typical targets, at up to 11.5 million. This is even greater than HER2, though far more tumor-specific.
The addition of naked anti-CanAg antibody to IKS04 significantly reduces MED in high- and very high-expressing models with minimal impact on the efficacy observed for the ADC in low-expressing models.
Importantly, this enhancement in efficacy does not negatively impact toxicity thresholds. In toxicology studies in rats and non-human primates, ultra-low DAR IKS04 is associated with a favorable, clinically desirable TI.
IKS04: a new approach for hard-to-treat GI cancers |
Highly potent proPBD payload with tumor-selective activation |
Highly tumor-specific, high density GI cancer target; CA242 |
Site-specific homogeneous conjugation |
Ultra-low DAR to overcome tumor penetration/ payload delivery challenges for high potency payloads |
Class leading TI |
Keep informed
Sign up for our latest programme news and insights