IKS014
IKS014 is currently in Phase 1 clinical trials in Australia for the treatment of patients with advanced HER2+ solid tumors.
Note: Patients are being recruited for Phase 2 trials in China (as FS-1502) for use in HER2+ solid tumors, and for a Phase 3 trial in China (as FS-1502) for the treatment of HER2+ advanced or metastatic breast cancer vs Kadcyla.
IKS014 development status
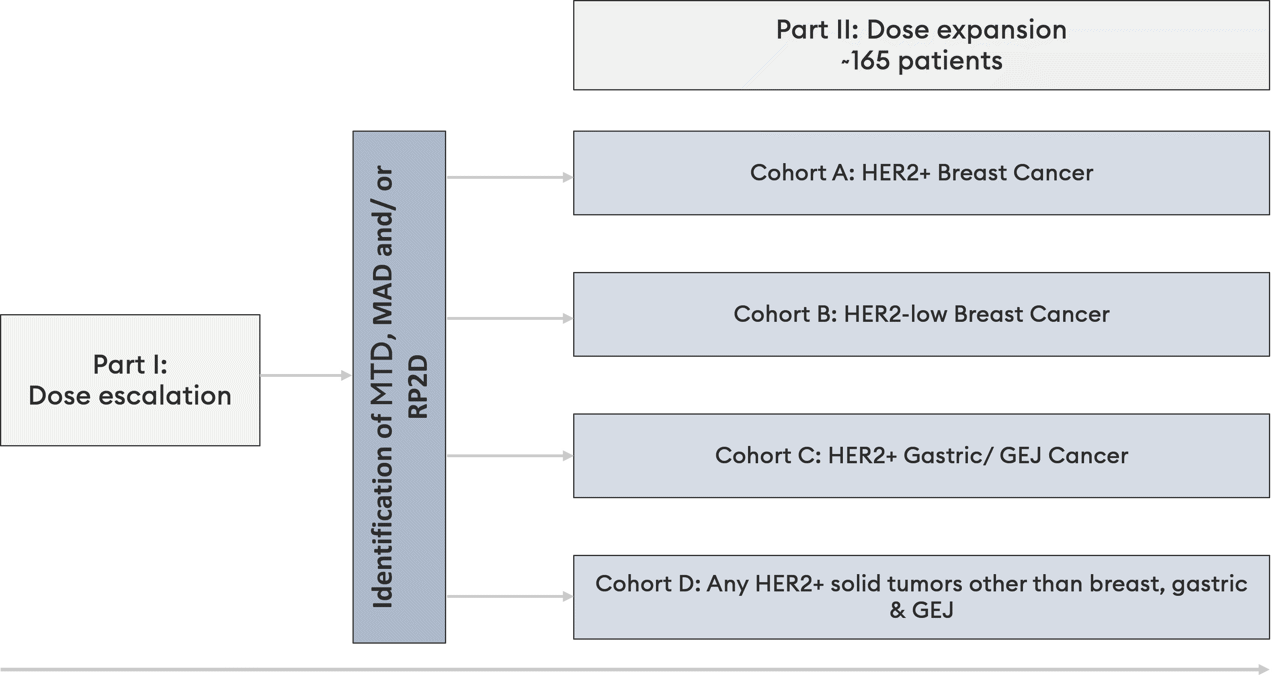
Clinical trial overview
Part I is a dose escalation 3+3 design study, with a primary objective of safety (MTD).
Part II is a dose expansion study in specified indications, with a primary objective of Objective Response Rate (ORR)
HER2 expression is defined as IHC3+, IHC2+/FISH+ or FISH+ and HER2-low expression is defined as IHC1+ or IHC2+/FISH-
Patients are currently being recruited for sites in Australia
Site locations
Australia
Australia Linear Clinical Research, 1 Hospital Avenue, B-Block, 1st Floor, Nedlands WA 6009, Australia
Peninsula & South Eastern Haematology and Oncology Group (PSEHOG), South Building, 5 Susono Way, Frankston VIC 3199
Frankston Hospital, 2 Hastings Rd, Frankston VIC 3199, Australia
Concord Repatriation General Hospital, Hospital Rd, Concord NSW 2139
Westmead Breast Cancer Institute, Block F/189 Cnr Hawkesbury & Darcy Rd, Westmead NSW 2145

Differentiation by design
A beta-glucuronide liker is used for tumor selective enzymatic payload release.
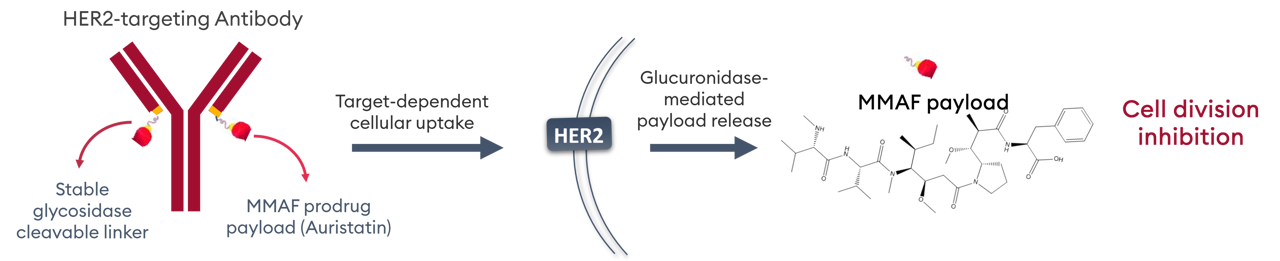
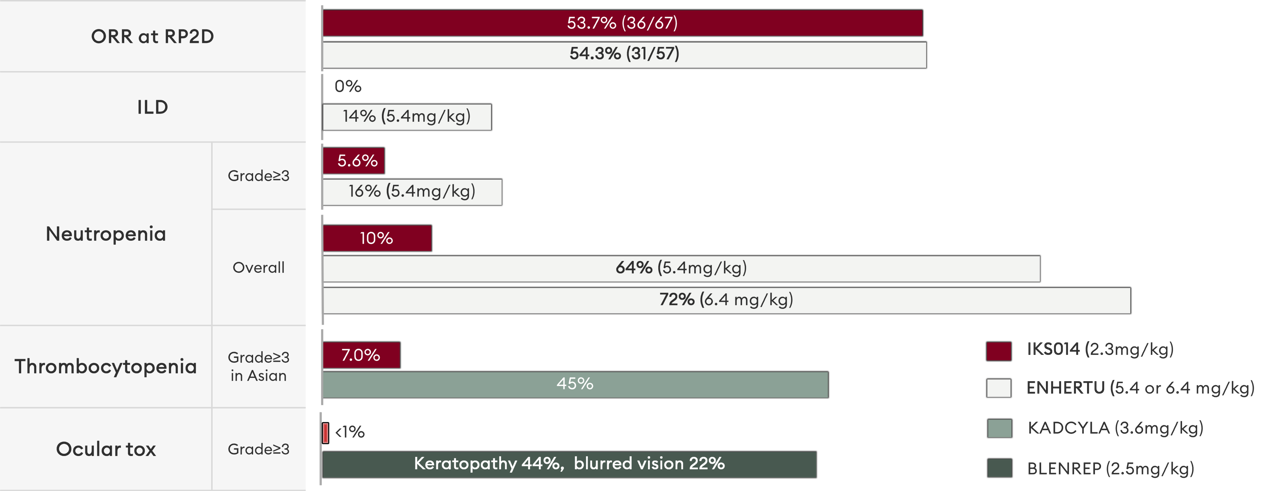
Tumor-selective payload activation drives a differentiated clinical profile
Compared with Enhertu and Kadcyla, there is significantly less neutropenia and thrombocytopenia and, importantly, a lack of dose-limiting ILD or respiratory disorders. ALso, unlike Blenrep - the only MMAF based ADC on market - FS-1502 is not associated with dose-limiting corneal toxicity or loss of visual acuity.
This enhanced safety profile does not come at the expense of efficacy - at the median follow-up of 5.2 months, FS-1502 showed a similar ORR in this patient population at a similar data cut-off point.
Iksuda has Best in class TI for HER2-directed therapies
Preclinical data has shown that IKS014 is associated with a significantly superior TI over Kadcyla and Enhertu.
Efficacy was similar or better than Enhertu and superior to Kadcyla in xenograft models for breast and gastric with moderate-to-high HER2 expression, whilst GLP toxicology confirmed a differentiated toxicity profile and an HNSTD of >12-fold the minimal effective dose (MED).
Preclinically, the advanced ADC design of IKS014 was shown to avoid dose-limiting ocular toxicities that are associated with MMAF-based ADCs, and respiratory, blood & lymphatic toxicities that are dose-limiting for other anti-HER2 ADCs including Enhertu.
| KadCYLA (T-DM1) | ENHERTU | XMT-1522 | IKS014 | |
| Company | Genetech/ Roche | Daiichi Sankyo/ AstraZeneca | Mersana/ Takeda | Iksuda |
| Payload (DAR) |
DM1 |
DXd (7.7) |
Auristatin D (15) |
MMAF (2) |
| MED (JIMT-1) | >20mg/kg | >10mg/kg | 1mg/kg | 1mg/kg |
| HNSTD | 30mg/kg | 30mg/kg | 2.5mg/kg | 12mg/kg |
| TI | <1.5 | <3 | 2.5 | 12 |
Downloads
0.26Mb PDF
0.25Mb PDF
Keep informed
Sign up for our latest programme news and insights